In clinical trials of adults with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC)
Significant response with Trulance vs placebo1,2,a
In Study 1 of patients with CIC, the percentage of overall efficacy respondersb (durable complete spontaneous bowel movement) was 21% for Trulance vs 10% for placebo (P<0.005).1 In Study 3 of patients with IBS-C, the percentage of overall efficacy respondersb (abdominal pain and stool frequency) was 30% for Trulance vs 18% for placebo (P<0.001).1,2
MORE
regular, well-formed BOWEL MOVEMENTS1–3,c
LESS IBS-C related
IBS-C related abdominal PAIN and BLOATING1,3,d
aTrulance-treated patients generally returned to baseline for these study endpoints during the post-treatment period.1
bIn Study 1 of CIC patients, a responder was defined as a patient who had at least 3 complete spontaneous bowel movements in a given week and an increase of at least 1 complete spontaneous bowel movement from baseline in the same week for at least 9 weeks out of the 12-week treatment period and at least 3 of the last 4 weeks of the study.1 In Study 3 of IBS-C patients, a responder was defined as a patient who met both the abdominal pain intensity and stool frequency responder criteria in the same week for at least 6 of the 12 treatment weeks, which were defined as an abdominal pain intensity responder (a patient who experienced a decrease in the weekly average score of worst abdominal pain in the past 24 hours [measured daily] of ≥30% compared with weekly baseline average) and stool frequency responder (a patient who experienced an increase of at least 1 complete spontaneous bowel movement per week from baseline).1
cIn 12-week clinical studies, more Trulance-treated patients had improvements in stool frequency (as measured by increased CSBMs, 21–48% Trulance vs 10–35% placebo) and consistency of bowel movements (as measured by mean increase in BSFS score, 1.5 Trulance vs 0.8–0.9 placebo).1,2,4,5
dAbdominal pain and constipation were components of the primary endpoints. Mean change from baseline in abdominal symptoms, including bloating, were measured as a secondary endpoint over 12 weeks in Phase III registrational trials. In clinical studies, more Trulance-treated patients were IBS-C abdominal pain responders (33–41%) compared to placebo (23–32%). Greater improvements in mean bloating score were also seen with Trulance (0.5–1.5) as compared to placebo (0.4–1.1).1,2,4,5
The efficacy and safety of Trulance was established in 4 clinical trials across 2 indications with more than 3100 patients1
Trulance was studied in two 12-week, double-blind, placebo-controlled, randomized, multicenter clinical studies in 1453 adult patients who met Rome III criteria for IBS-C and received placebo (n=729) or Trulance 3 mg (n=724) once daily without respect to food. Efficacy was assessed using information provided by patients on a daily basis through an electronic phone diary system.1
Patients included in clinical trials:
Study 3 mean baseline characteristics for patients on Trulance vs placebo
CSBMse/week2
0.2 vs 0.2
Abdominal pain2,f
5.9 vs 6.1
Stool consistency2,g
2.0 vs 2.1
Bloating2,f
6.4 vs 6.5
eA CBSM, or a complete spontaneous bowel movement, is an SBM with the sense of complete evacuation; an SBM is a spontaneous bowel movement that occurs in the absence of laxative use within the previous 24 hours.1,2
fAbdominal pain and bloating were assessed on a 11-point numeric rating scale: 0 (none) to 10 (worst possible). An abdominal pain intensity responder required a decrease in the weekly worst abdominal pain intensity (WAPI) score in the past 24 hours of ≥30% compared with weekly baseline average for at least 6 of the 12 treatment weeks.1,2
gMeasured on 7-point Bristol Stool Form Scale: 1=separate, hard lumps, like nuts (hard to pass); 7=watery, no solid pieces (entirely liquid).2
Study 4 is a similar 12-week, double-blind, placebo-controlled, randomized, multicenter clinical study of adult patients who met Rome III criteria for IBS-C and were randomized 1:1 to either placebo or Trulance 3 mg.1 It demonstrated similar baseline characteristics for patients treated with Trulance or placebo, respectively: 0.3 vs 0.2 CSBMs/week, 6.6 vs 6.4 abdominal pain, a stool consistency of 1.9 vs 2.0, and a bloating score of 6.6 vs 6.5.1,2
Trulance was studied in two 12-week, double-blind, placebo-controlled, randomized, multicenter clinical studies in 1453 adult patients who met Rome III criteria for IBS-C and received placebo (n=729) or Trulance 3 mg (n=724) once daily without respect to food. Efficacy was assessed using information provided by patients on a daily basis through an electronic phone diary system.1
Patients included in clinical trials:
Study 3 mean baseline characteristics for patients on Trulance vs placebo
CSBMse/week2
0.2 vs 0.2
Abdominal pain2,f
5.9 vs 6.1
Stool consistency2,g
2.0 vs 2.1
Bloating2,f
6.4 vs 6.5
eA CBSM, or a complete spontaneous bowel movement, is an SBM with the sense of complete evacuation; an SBM is a spontaneous bowel movement that occurs in the absence of laxative use within the previous 24 hours.1,2
fAbdominal pain and bloating were assessed on a 11-point numeric rating scale: 0 (none) to 10 (worst possible). An abdominal pain intensity responder required a decrease in the weekly worst abdominal pain intensity (WAPI) score in the past 24 hours of ≥30% compared with weekly baseline average for at least 6 of the 12 treatment weeks.1,2
gMeasured on 7-point Bristol Stool Form Scale: 1=separate, hard lumps, like nuts (hard to pass); 7=watery, no solid pieces (entirely liquid).2
Study 4 is a similar 12-week, double-blind, placebo-controlled, randomized, multicenter clinical study of adult patients who met Rome III criteria for IBS-C and were randomized 1:1 to either placebo or Trulance 3 mg.1 It demonstrated similar baseline characteristics for patients treated with Trulance or placebo, respectively: 0.3 vs 0.2 CSBMs/week, 6.6 vs 6.4 abdominal pain, a stool consistency of 1.9 vs 2.0, and a bloating score of 6.6 vs 6.5.1,2
Trulance was studied in two 12-week, double-blind, placebo-controlled, randomized, multicenter clinical studies in 1775 adult patients who met Rome III criteria for CIC and were randomized 1:1 to either placebo (n=892) or Trulance 3 mg (n=883) once daily without respect to food. Efficacy was assessed using information provided by patients on a daily basis in an electronic diary.1
Patients included in clinical trials:
Study 1 baseline characteristics for patients on Trulance vs placebo
CSBMse/week4
0.3 vs 0.4
SBMse/week4
2.0 vs 2.2
Stool consistency4,g
2.5 vs 2.6
Straining4,h
2.3 vs 2.3
eA CBSM, or a complete spontaneous bowel movement, is an SBM with the sense of complete evacuation; an SBM is a spontaneous bowel movement that occurs in the absence of laxative use within the previous 24 hours.1,4
gMeasured on 7-point Bristol Stool Form Scale: 1=separate, hard lumps, like nuts (hard to pass); 7=watery, no solid pieces (entirely liquid).4
hStraining was reported in Daily Symptom Diary, measured on 5-point Likert Scale: 0=none; 4=very severe.4
Study 2 is a similar 12-week, double-blind, placebo-controlled, randomized, multicenter clinical study of adult patients who met Rome III criteria for CIC and were randomized 1:1 to either placebo or Trulance 3 mg.5 It demonstrated similar baseline characteristics for patients on Trulance (n=430) or placebo (n=440), respectively, with 0.28 vs 0.31 mean CSBMs/week, 1.79 vs 1.55 SBMs/week, a stool consistency of 2.16 vs 2.35, and a straining score of 2.45 vs 2.41.1,5
Trulance was studied in two 12-week, double-blind, placebo-controlled, randomized, multicenter clinical studies in 1775 adult patients who met Rome III criteria for CIC and were randomized 1:1 to either placebo (n=892) or Trulance 3 mg (n=883) once daily without respect to food. Efficacy was assessed using information provided by patients on a daily basis in an electronic diary.1
Patients included in clinical trials:
Study 1 baseline characteristics for patients on Trulance vs placebo
CSBMse/week4
0.3 vs 0.4
SBMse/week4
2.0 vs 2.2
Stool consistency4,g
2.5 vs 2.6
Straining4,h
2.3 vs 2.3
eA CBSM, or a complete spontaneous bowel movement, is an SBM with the sense of complete evacuation; an SBM is a spontaneous bowel movement that occurs in the absence of laxative use within the previous 24 hours.1,4
gMeasured on 7-point Bristol Stool Form Scale: 1=separate, hard lumps, like nuts (hard to pass); 7=watery, no solid pieces (entirely liquid).4
hStraining was reported in Daily Symptom Diary, measured on 5-point Likert Scale: 0=none; 4=very severe.4
Study 2 is a similar 12-week, double-blind, placebo-controlled, randomized, multicenter clinical study of adult patients who met Rome III criteria for CIC and were randomized 1:1 to either placebo or Trulance 3 mg.5 It demonstrated similar baseline characteristics for patients on Trulance (n=430) or placebo (n=440), respectively, with 0.28 vs 0.31 mean CSBMs/week, 1.79 vs 1.55 SBMs/week, a stool consistency of 2.16 vs 2.35, and a straining score of 2.45 vs 2.41.1,5
Strong recommendation from ACG Clinical Guideline6
The 2020 American College of Gastroenterology (ACG) Clinical Guideline gave Trulance, as a guanylate cyclase-C (GC-C) agonist, a strong recommendation with a high quality of evidence for the treatment of global IBS-C symptoms6,*


The 2020 American College of Gastroenterology (ACG) Clinical Guideline gave Trulance, as a guanylate cyclase-C (GC-C) agonist, a strong recommendation with a high quality of evidence for the treatment of global IBS-C symptoms6,*
Effective for relieving overall and individual symptoms of IBS-C
Responses develop quickly and are maintained over time
Diarrhea is the most common adverse event experienced, but it is well tolerated, and discontinuation rates due to diarrhea are low
*Strength of recommendation: Strong=Most patients should receive the recommended course of action. Quality of evidence: High=The estimate of effect is unlikely to change with new data.6
Clinical trial results
See how Trulance helped IBS-C and CIC patients across the
following efficacy measures
Trulance produced a durable response1
Significant response with Trulance vs placebo1,i
Overall efficacy responders (abdominal pain + stool frequency)1,2
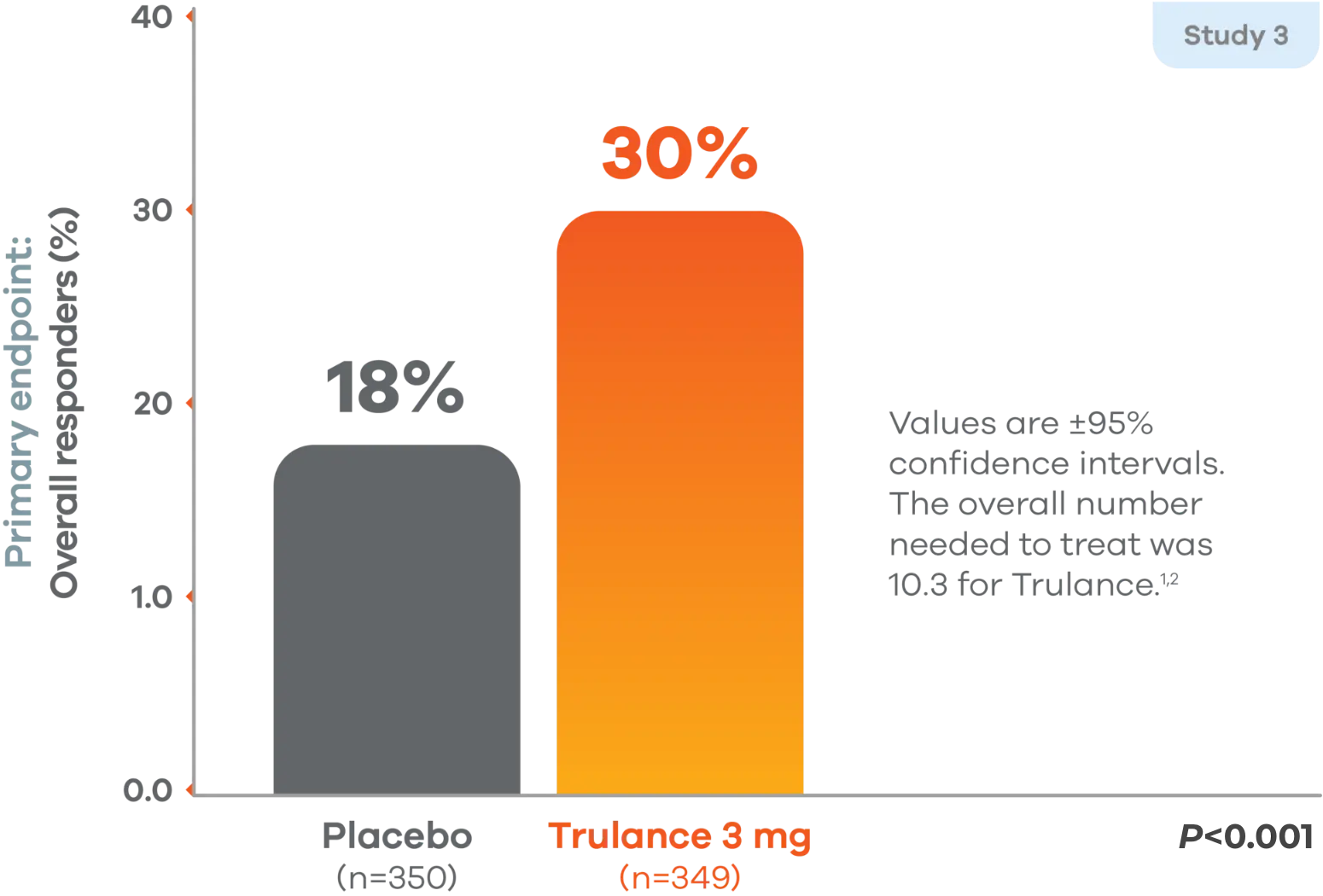

Primary endpoint: A responder was defined as a patient who met both the abdominal pain intensity and stool frequency responder criteria in the same week for at least 6 of the 12 treatment weeks, which were defined as an abdominal pain intensity responder (a patient who experienced a decrease in the weekly average score of worst abdominal pain in the past 24 hours [measured daily] of ≥30% compared with weekly baseline average) and stool frequency responder (a patient who experienced an increase of at least 1 CSBM per week from baseline).1
Study 4 demonstrated similar numbers of overall efficacy responders: 21% found response on Trulance vs 14% on placebo (P=0.009).1,2
In both studies, the proportion of responders who were also weekly responders for at least 2 of the 4 treatment weeks in month 3, the last month of treatment, was greater in the Trulance groups compared to placebo. Abdominal pain was assessed on a rating scale of 0 (none) to 10 (worst possible).1,2
iTrulance-treated patients generally returned to baseline for these study endpoints during the post-treatment period.1
Durable response with Trulance was demonstrated vs placebo1,4,5,j
Study 1 durable overall CSBM responders
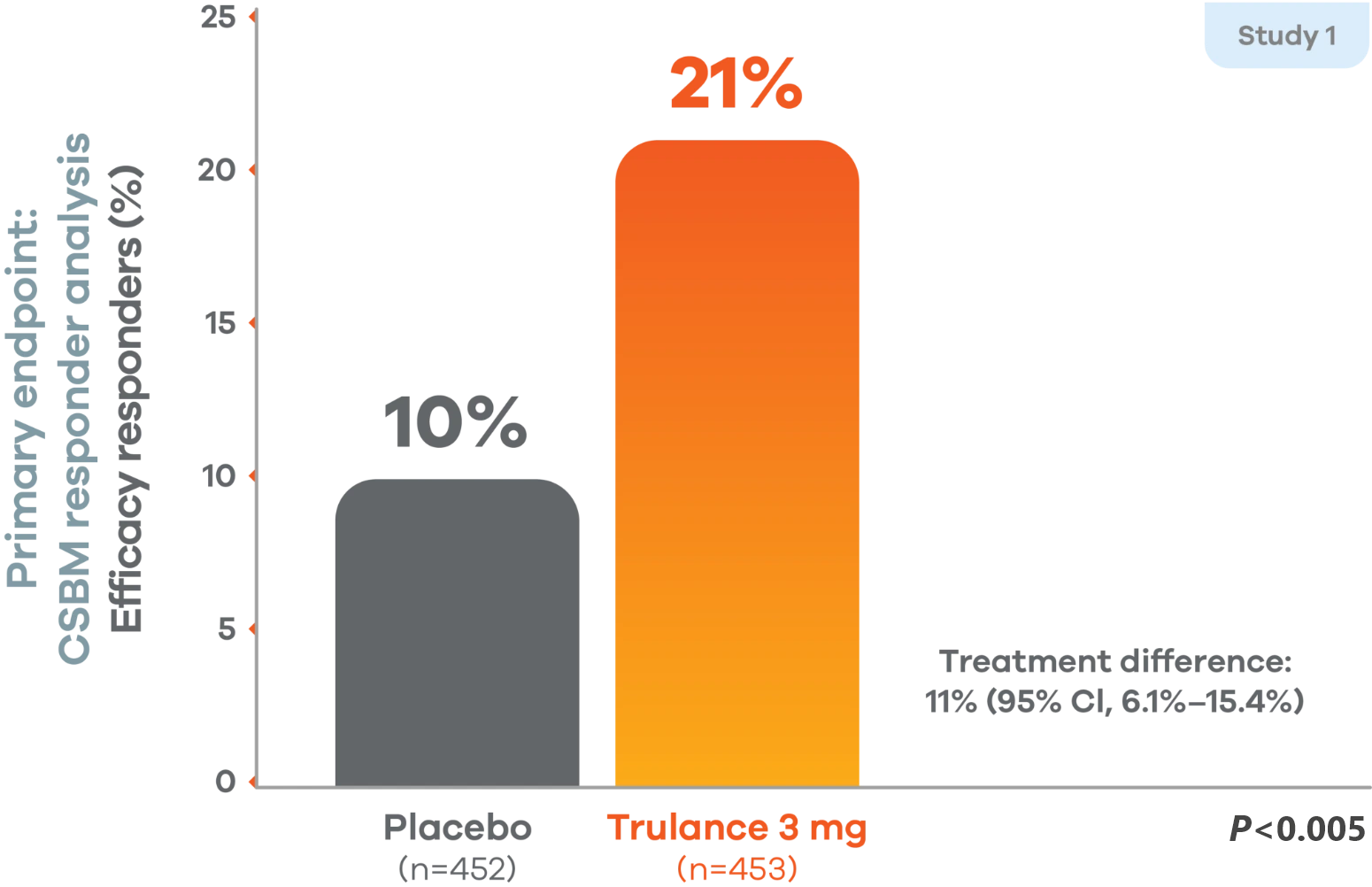

There was a significantly greater percentage of efficacy responders (durable CSBM) in the Trulance group than in the placebo group.1,4
A responder was defined as a patient who had at least 3 complete spontaneous bowel movements in a given week and an increase of at least 1 complete spontaneous bowel movement from baseline in the same week for at least 9 weeks out of the 12-week treatment period and at least 3 of the last 4 weeks of the study.1
Study 2 demonstrated similar numbers of responders for complete spontaneous bowel movement, with 21% of patients showing a response to Trulance (n=430) vs 13% with placebo (n=440).1,5
jTrulance-treated patients generally returned to baseline for these study endpoints during the post-treatment period.1
Significant reduction in abdominal pain after first week of treatment sustained through 12-week treatment period1,3
IBS-C
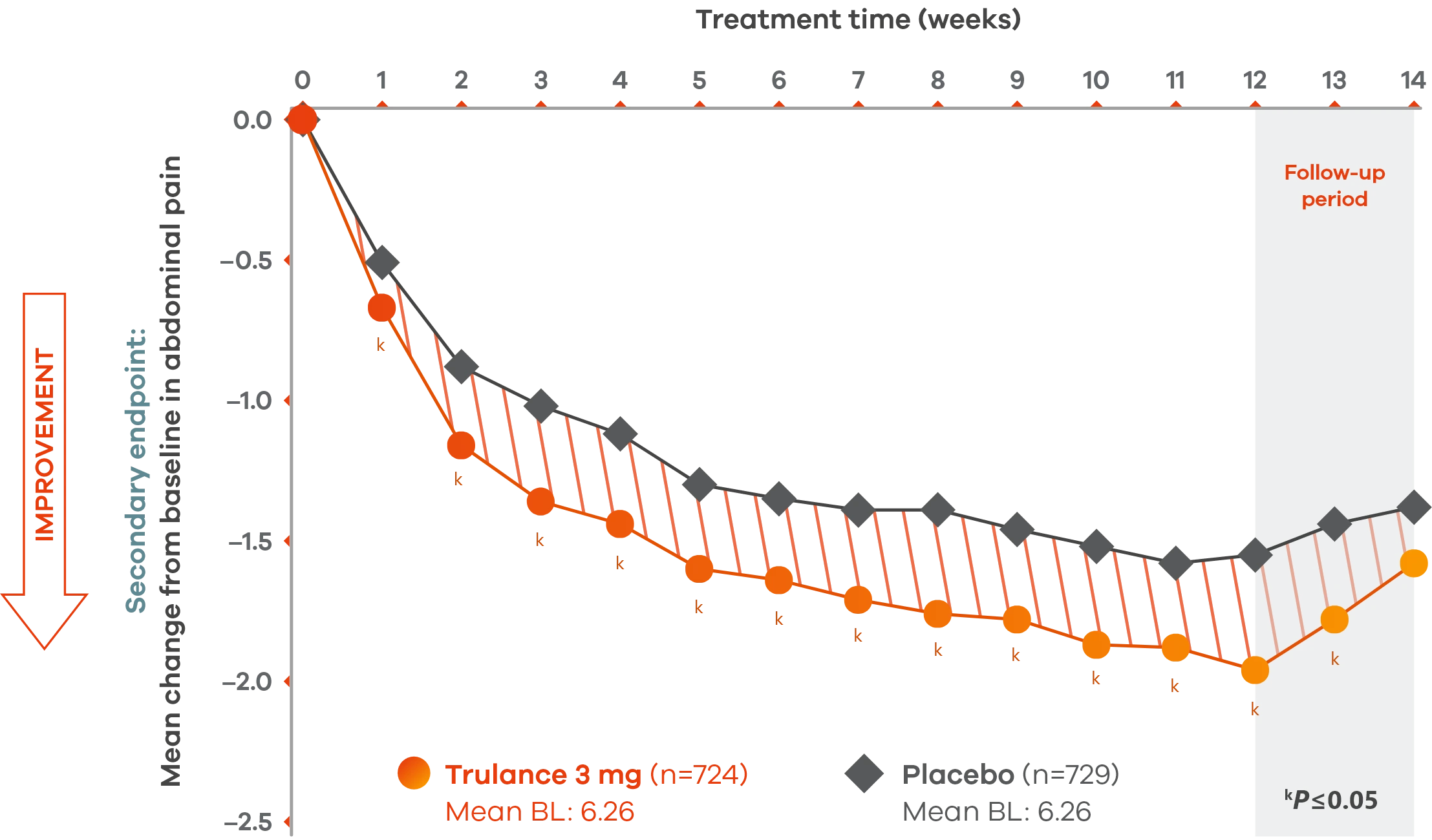

Pooled analysis of both Phase III IBS-C trials.
A pain responder required a decrease in the worst abdominal pain intensity (WAPI) score of ≥30% compared with weekly baseline average for at least 6 of the 12 treatment weeks.1
The severity of abdominal pain was assessed on an 11-point numeric rating scale from 0 (none) to 10 (worst possible).1
Trulance-treated patients generally returned to baseline for these study endpoints during the post-treatment period.1
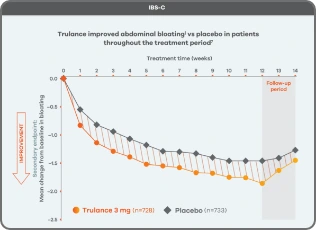
Improvement in key abdominal symptoms, including bloating7
IBS-C
Trulance improved abdominal bloatingl vs placebo in patients throughout the treatment period7
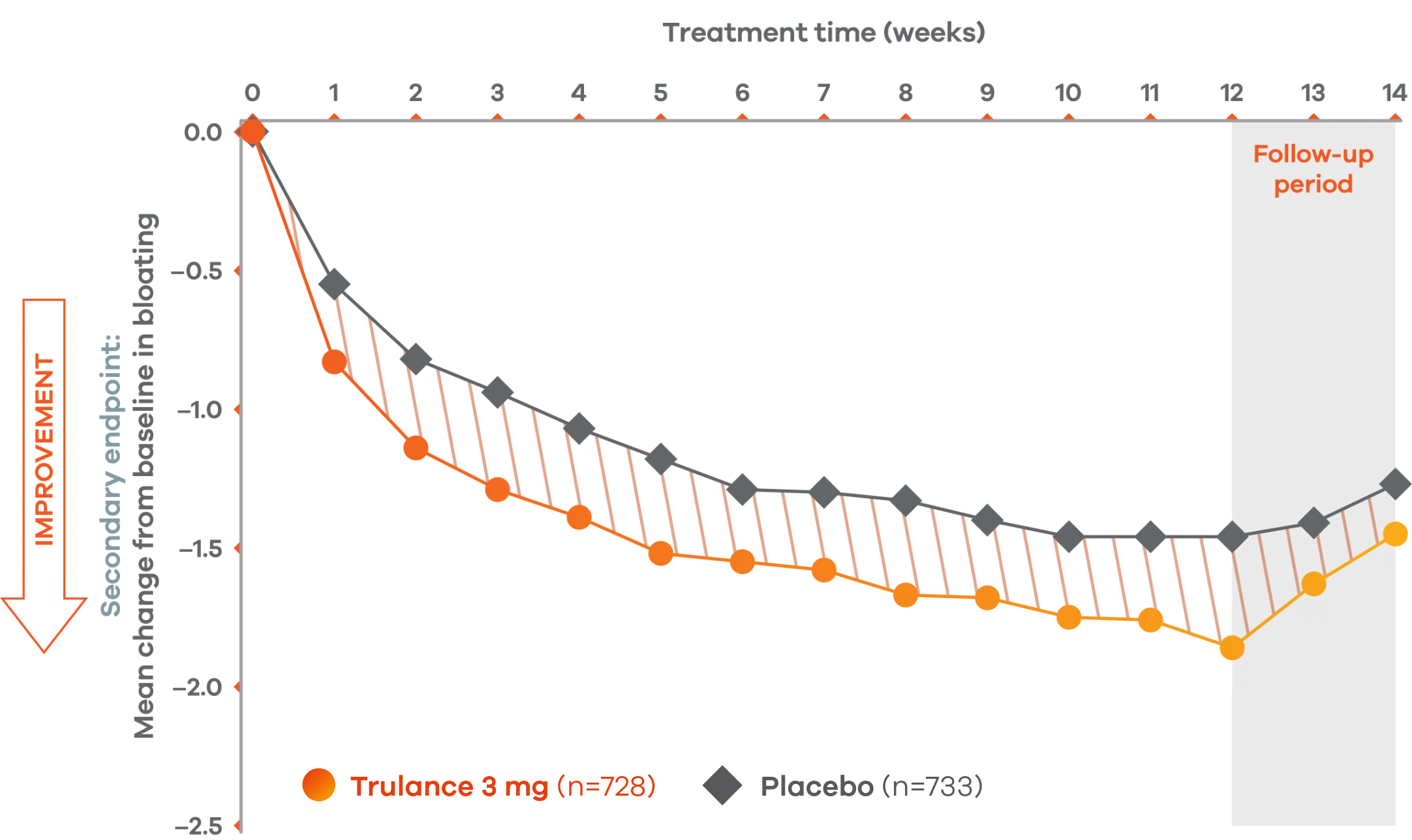
An integrated analysis of IBS-C Study 3 and 4. The severity of each symptom was assessed on an 11-point numeric rating scale from 0 (none) to 10 (worst possible) across 12 weeks.2
lMean change from baseline in abdominal symptoms were measured as a secondary endpoint over 12 weeks in Phase III registrational trials.
Because these individual component analyses were not adjusted for multiplicity, individual component results need cautious interpretation and could represent chance findings.
Trulance improved abdominal crampingl in patients throughout the treatment period7
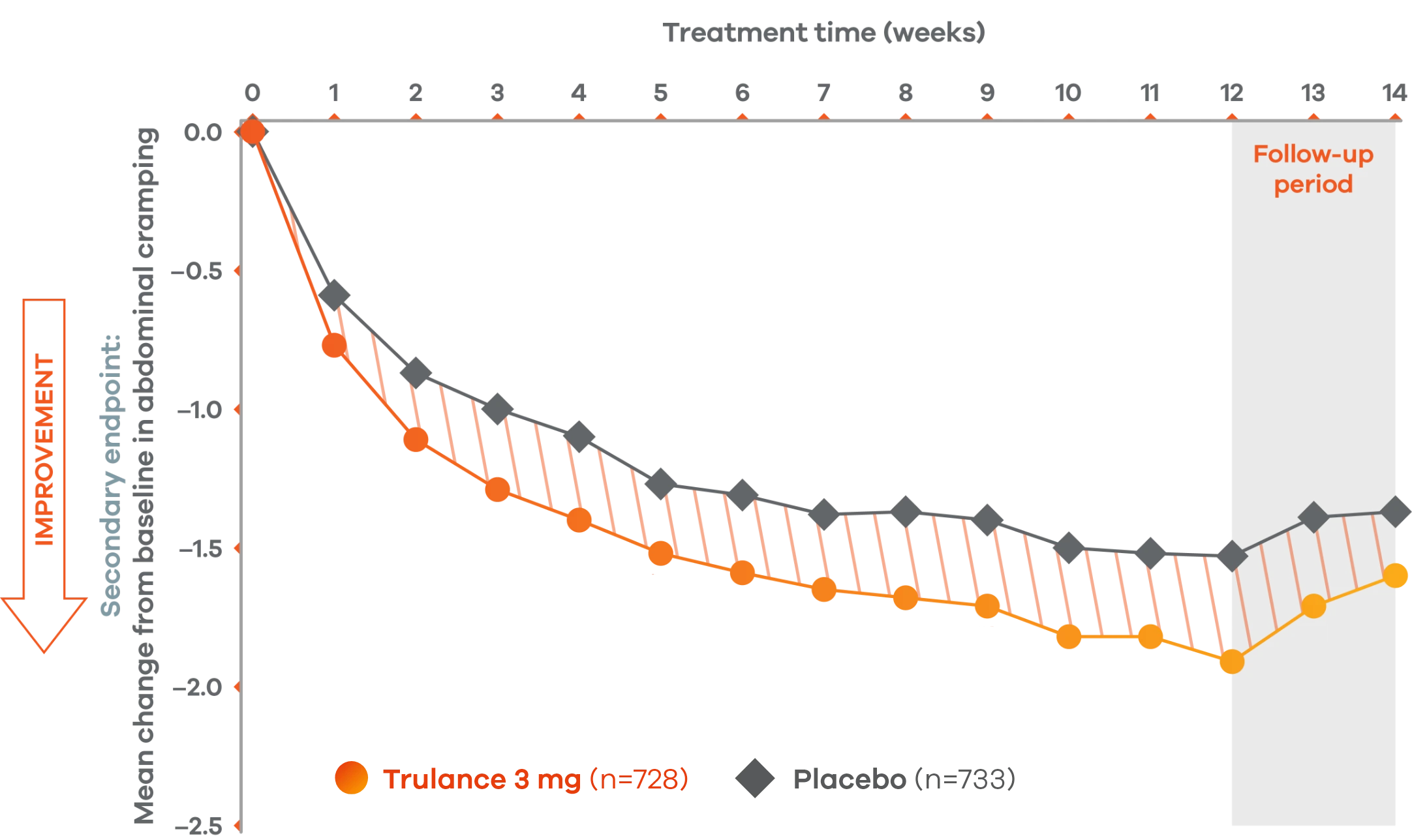
An integrated analysis of IBS-C Study 3 and 4. The severity of each symptom was assessed on an 11-point numeric rating scale from 0 (none) to 10 (worst possible) across 12 weeks.2
lMean change from baseline in abdominal symptoms were measured as a secondary endpoint over 12 weeks in Phase III registrational trials.
Because these individual component analyses were not adjusted for multiplicity, individual component results need cautious interpretation and could represent chance findings.
Trulance improved abdominal discomfortl in patients throughout the treatment period7
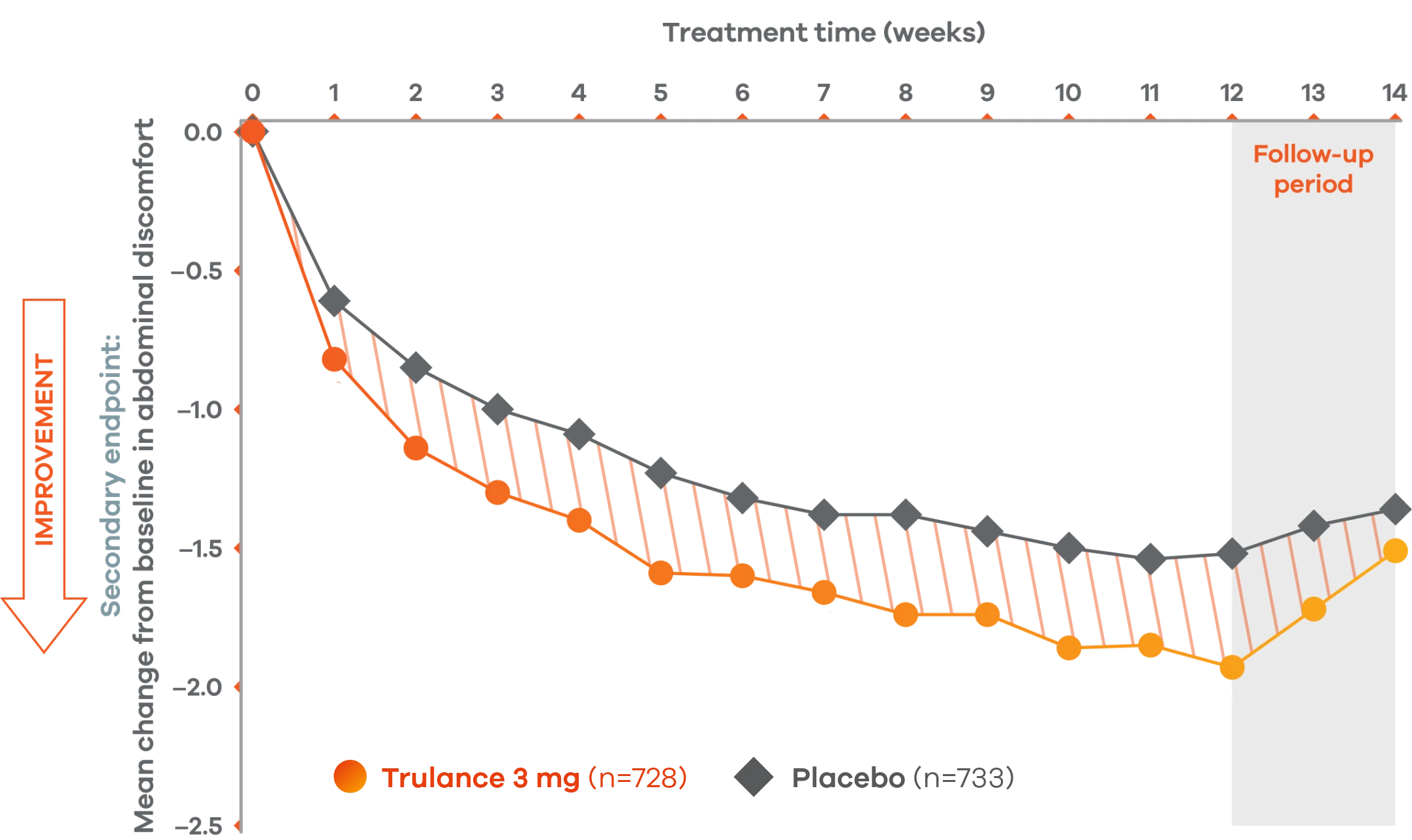
An integrated analysis of IBS-C Study 3 and 4. The severity of each symptom was assessed on an 11-point numeric rating scale from 0 (none) to 10 (worst possible) across 12 weeks.2
lMean change from baseline in abdominal symptoms were measured as a secondary endpoint over 12 weeks in Phase III registrational trials.
Because these individual component analyses were not adjusted for multiplicity, individual component results need cautious interpretation and could represent chance findings.
Trulance improved abdominal fullnessl in patients throughout the treatment period7
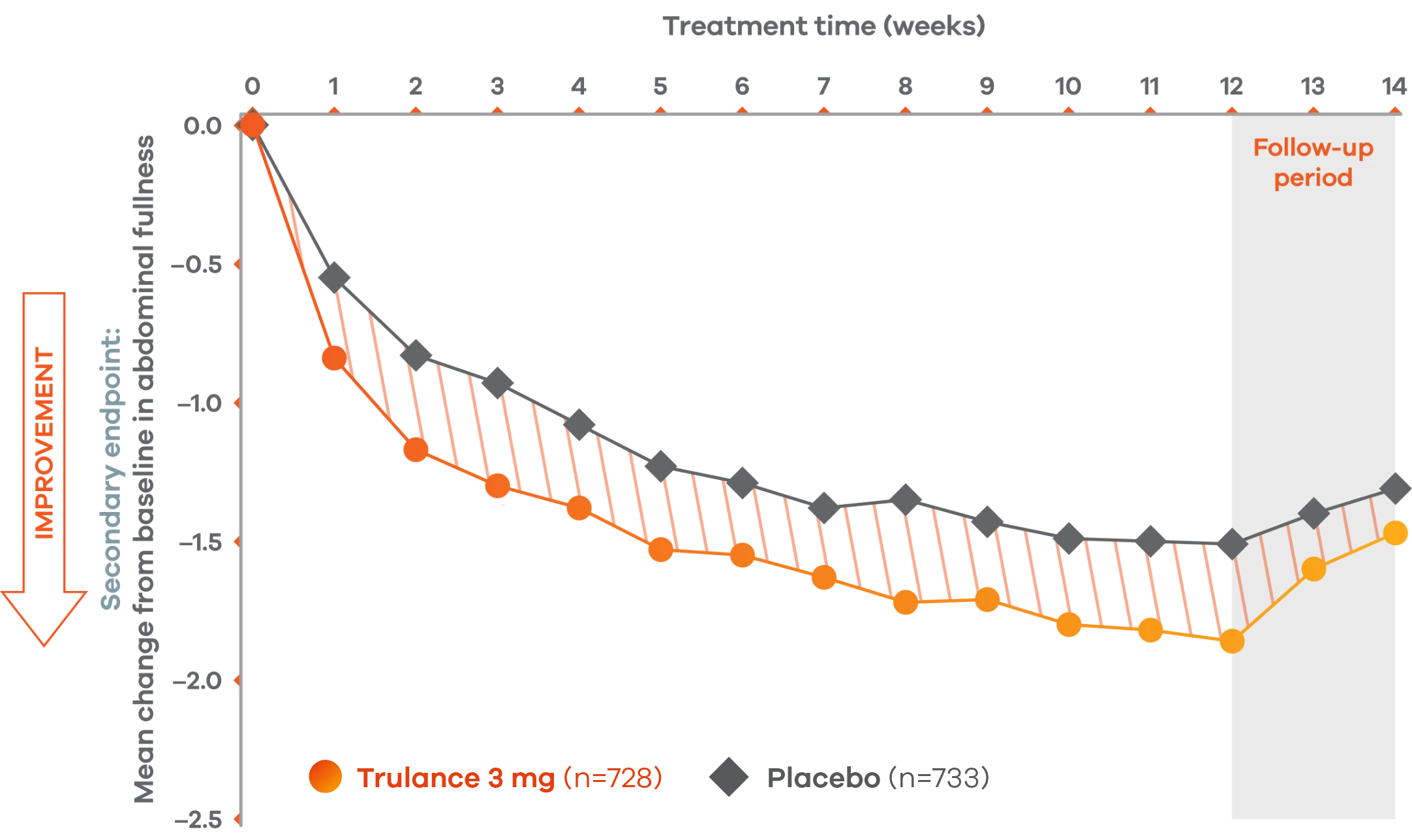
An integrated analysis of IBS-C Study 3 and 4. The severity of each symptom was assessed on an 11-point numeric rating scale from 0 (none) to 10 (worst possible) across 12 weeks.2
lMean change from baseline in abdominal symptoms were measured as a secondary endpoint over 12 weeks in Phase III registrational trials.
Because these individual component analyses were not adjusted for multiplicity, individual component results need cautious interpretation and could represent chance findings.
Trulance delivered results within 24 hours
More patients with IBS-C experienced spontaneous bowel movements within 24 hours vs placebo2
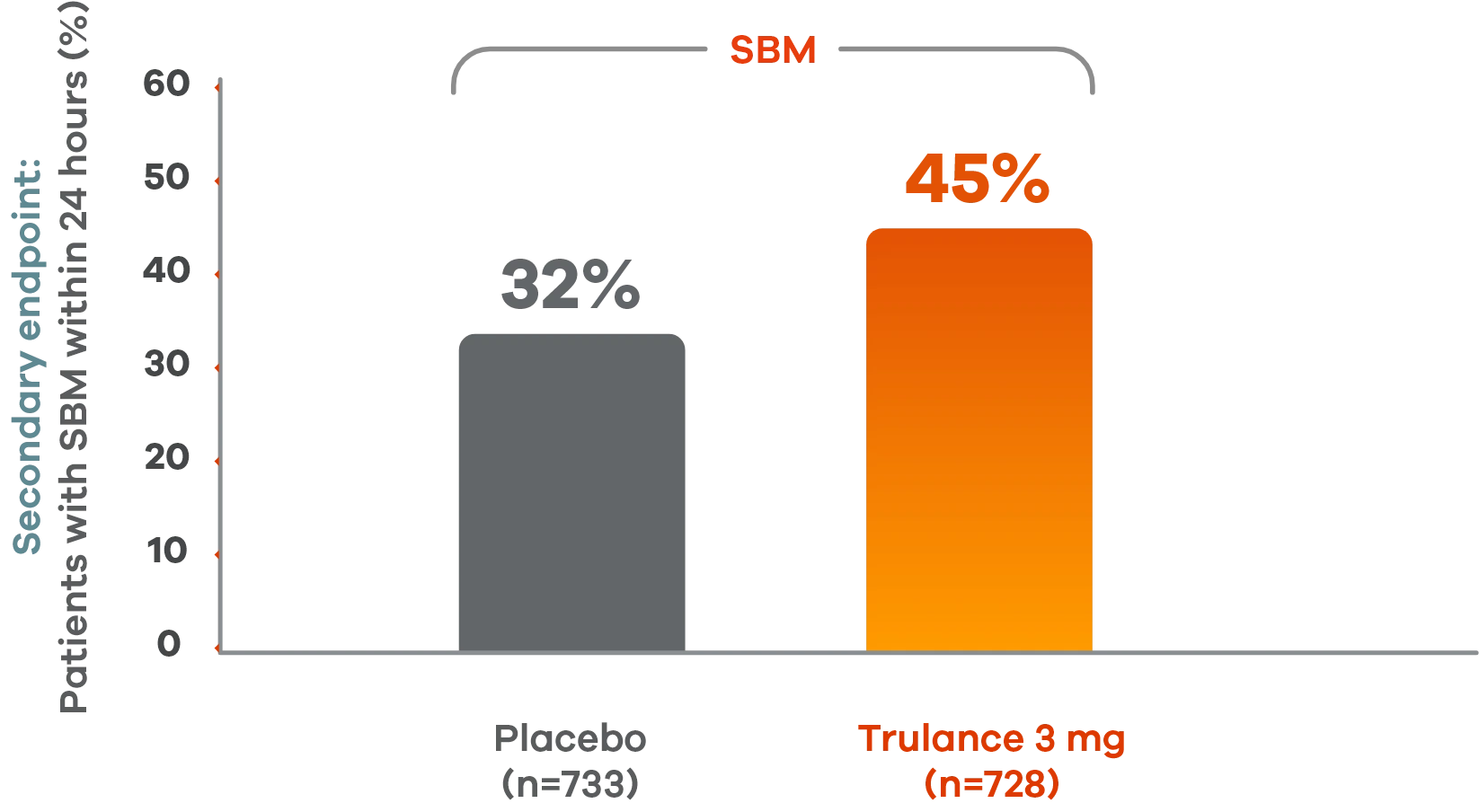

Pooled analysis of both Phase III IBS-C trials.
This analysis was not adjusted for multiplicity and should be interpreted with caution.
More patients experienced CSBMs and SBMs within 24 hours vs placebo4
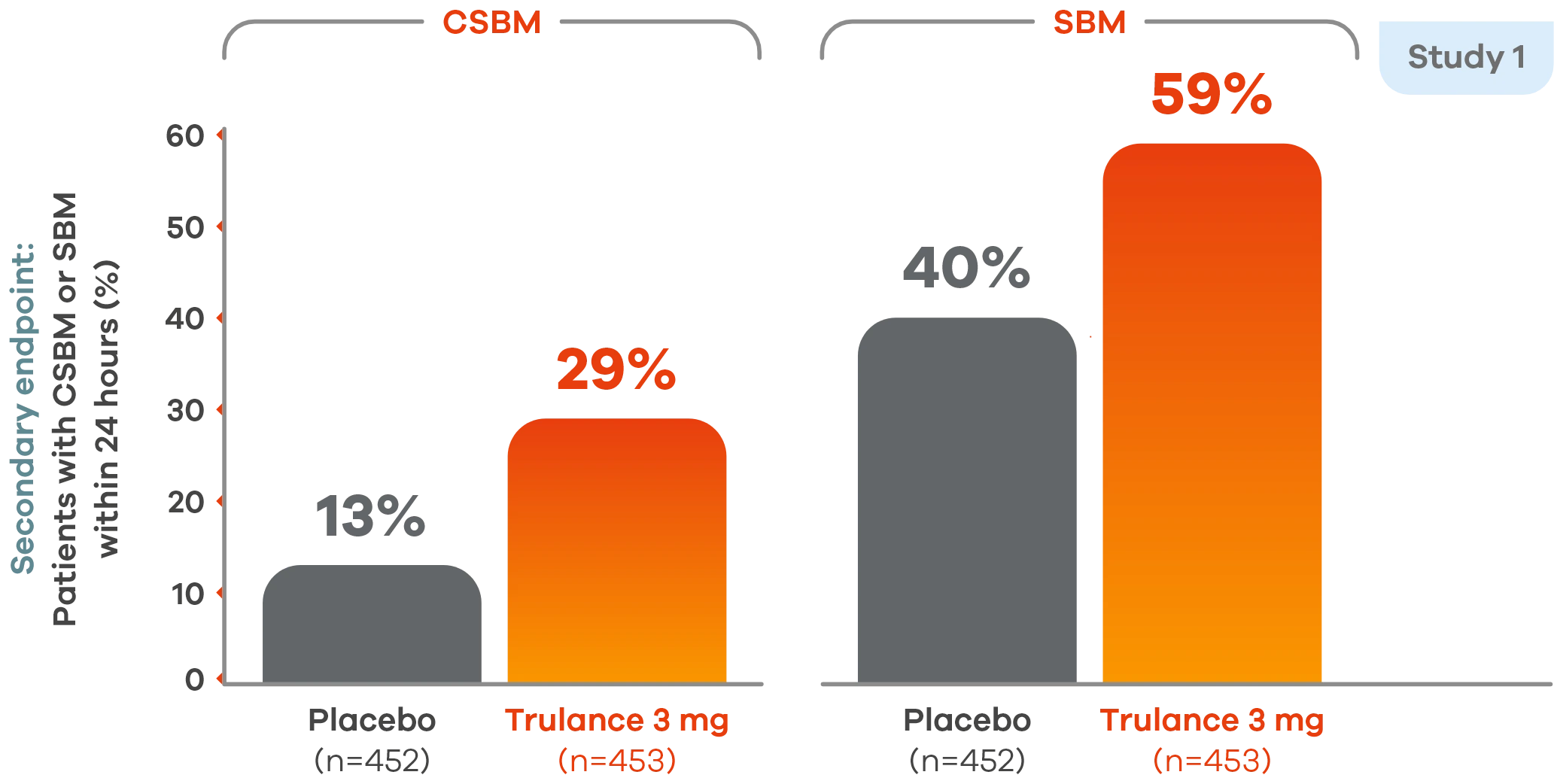

Study 2 demonstrated an improvement in the percentage of patients having CSBMs within 24 hours of the first dose of Trulance vs placebo, 21.4% vs 12.1%.5
Similar results were seen with SBMs within 24 hours of the first dose vs placebo, 43.6% vs 36.4%.5
This analysis was not adjusted for multiplicity and should be interpreted with caution.
Trulance significantly improved stool frequency4,7
CIC
Significant improvement in CSBM weekly frequency
3 at week 12 vs 0.3 at baseline4,7
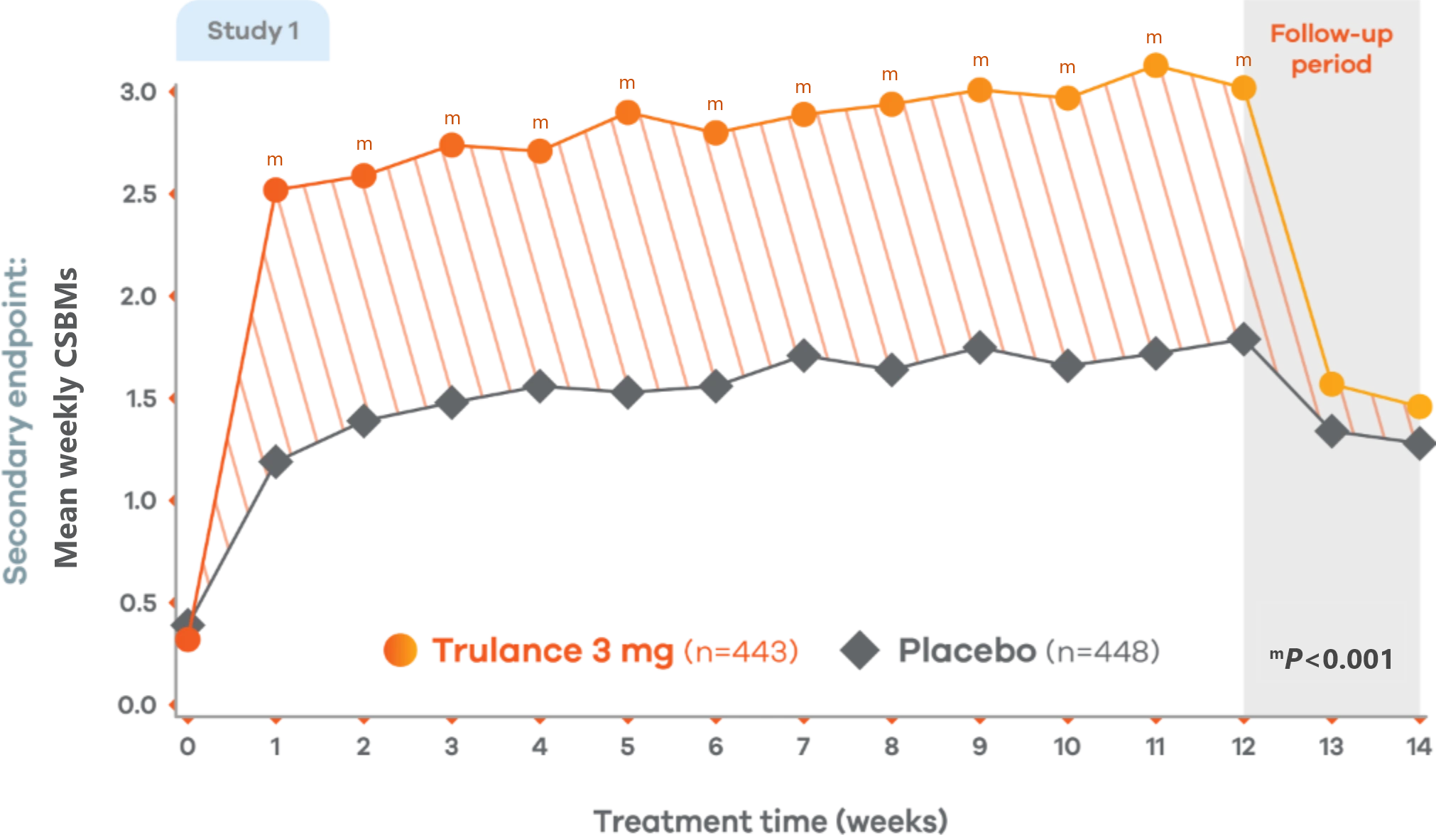

Changes from baseline in weekly CSBM frequency in Study 1. Values are least squares (LS) mean.4
Trulance-treated patients generally returned to baseline for these study endpoints during the post-treatment period.
Study 2 also demonstrated a similar change in mean weekly CSBM frequency from baseline through Week 12 for Trulance vs placebo.5
CIC
Significant improvement in SBM weekly frequency
More than 5 at week 12 vs 2 at baseline4,7
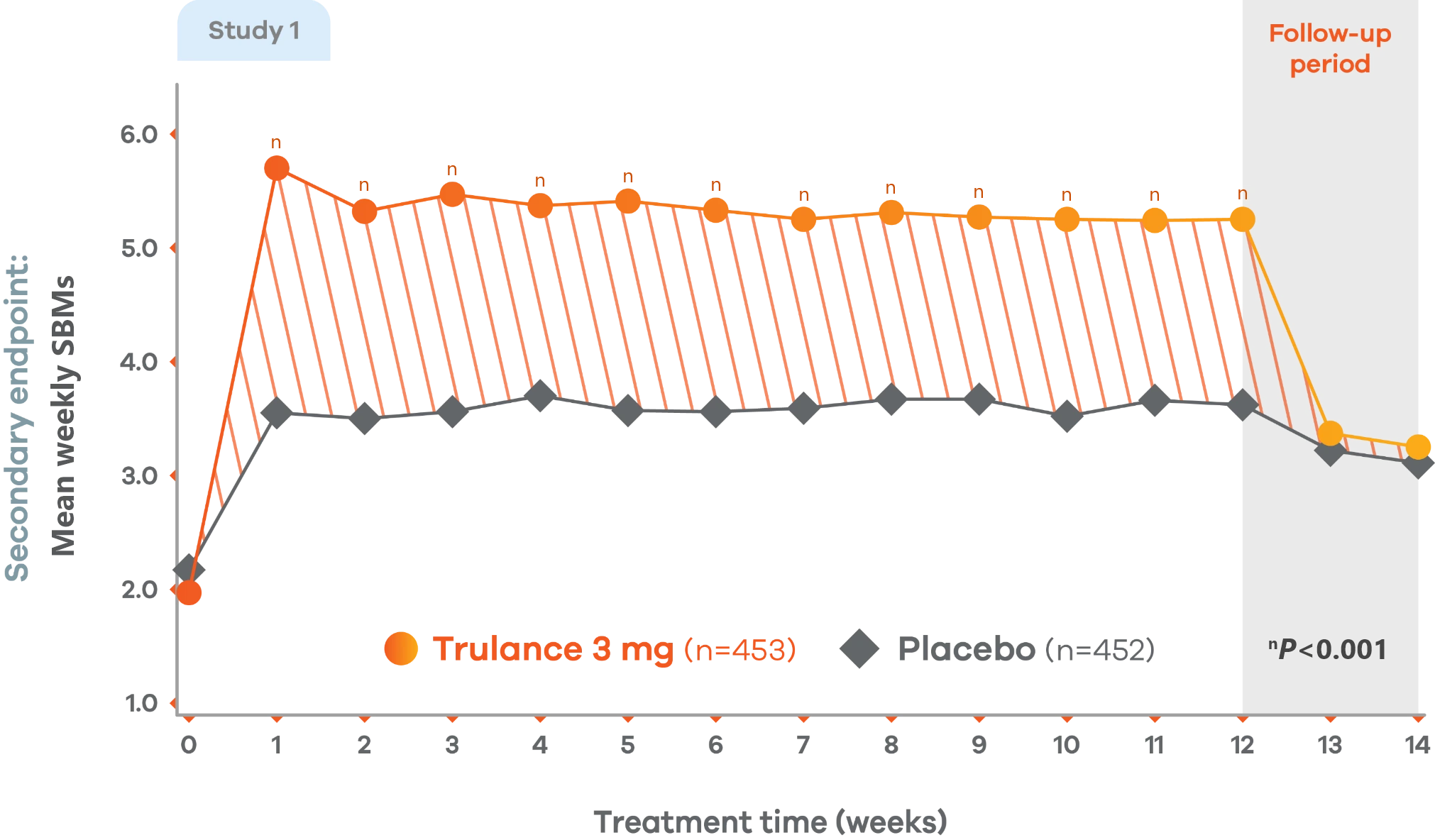

Changes from baseline in mean weekly SBM frequency. Values are LS mean.4
Trulance-treated patients generally returned to baseline for these study endpoints during the post-treatment period.
Study 2 demonstrated a similar change from baseline in mean weekly SBM frequency from baseline through week 12 for Trulance vs placebo.5
Trulance improved stool consistency
Consistent improvements in stool consistency in both IBS-C studies vs placebo2
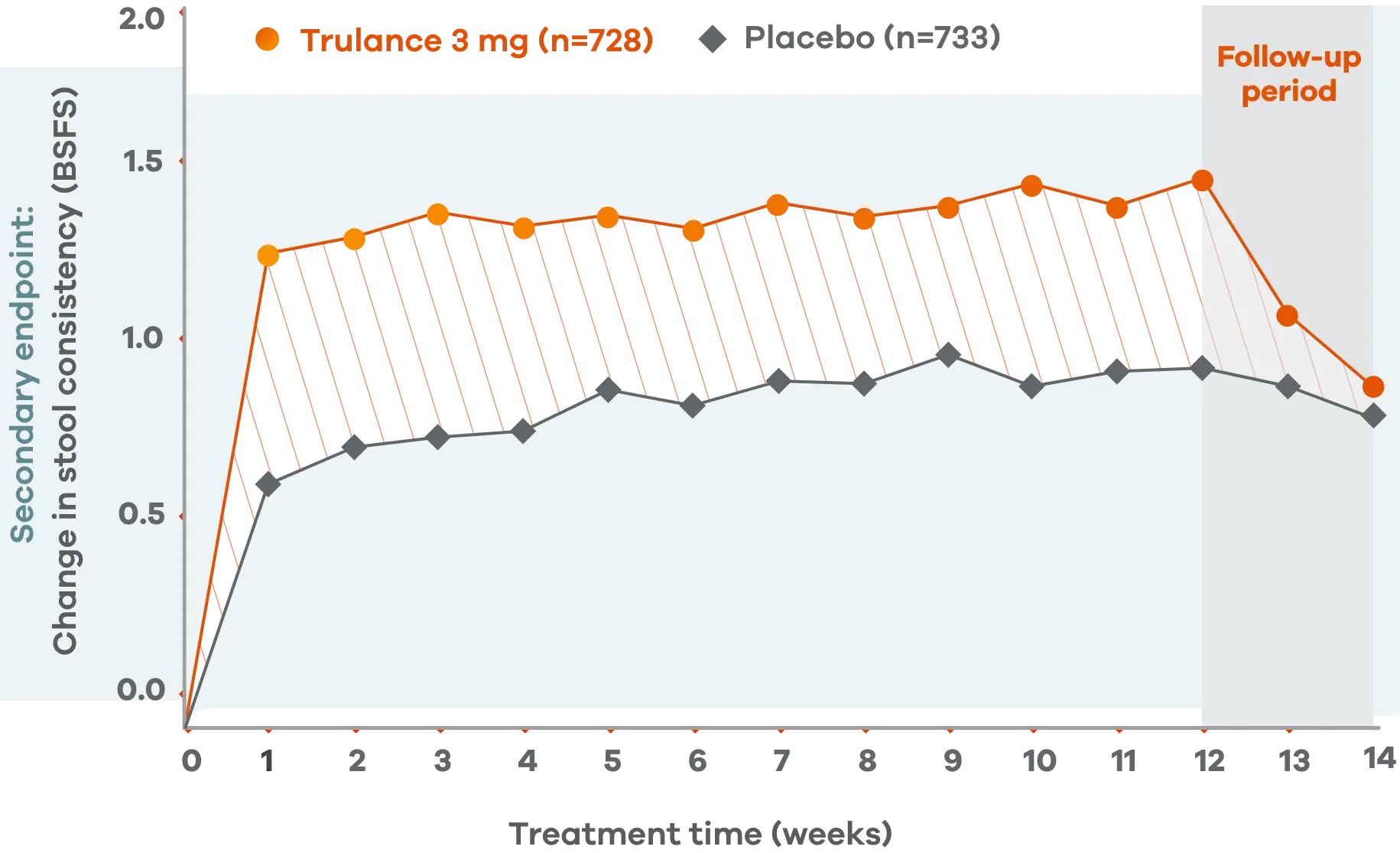

An integrated analysis of IBS-C Studies 3 and 4.2
Change from baseline in stool consistency by time point. Values are LS mean ± standard error.2
This analysis was not adjusted for multiplicity and should be interpreted with caution.
Patients on Trulance achieved and maintained a mean BSFS score of ~44,o
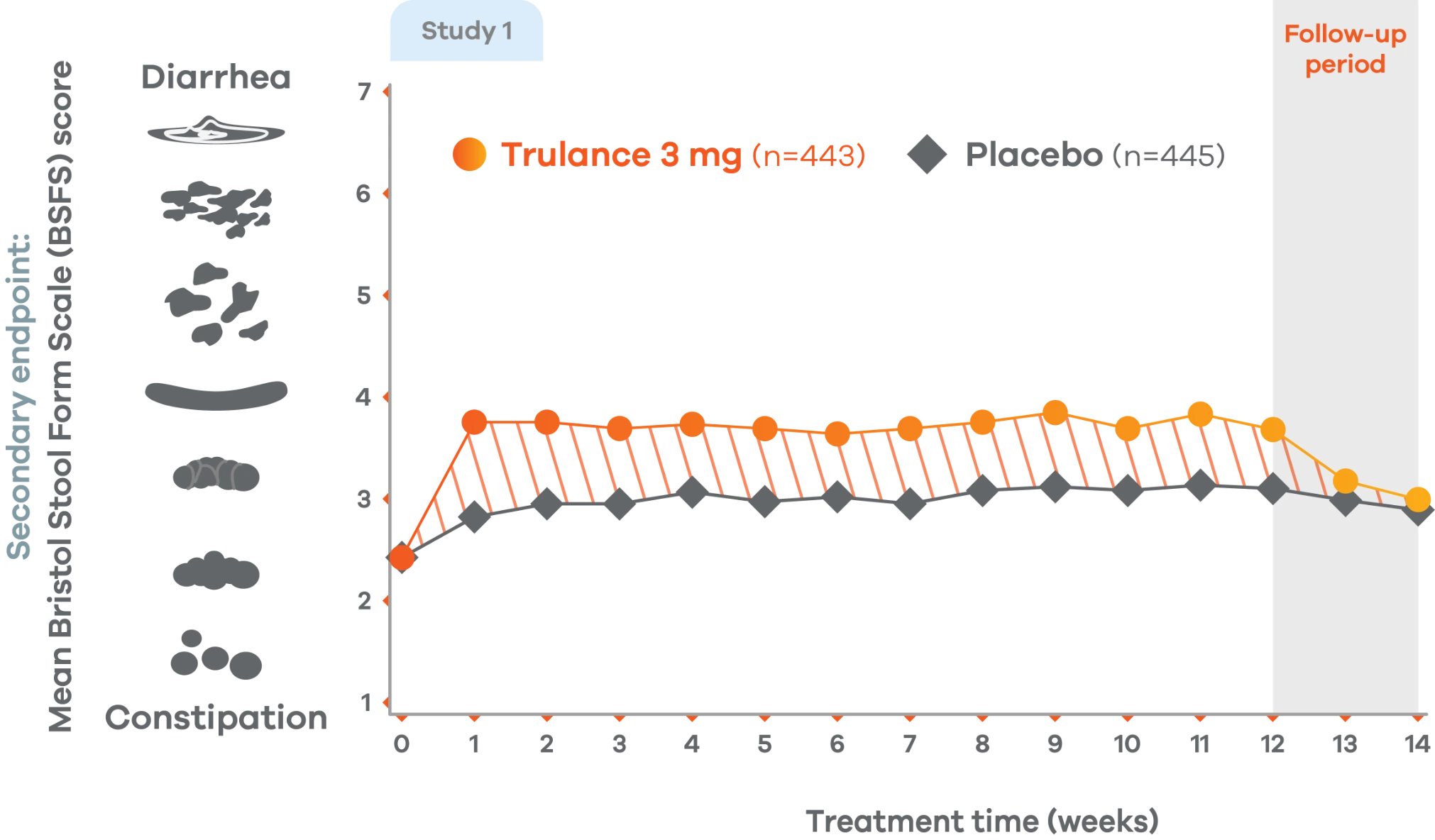

oStool consistency was measured in weekly BSFS scores; values are LS mean.
Study 2 demonstrated a similar change in stool consistency with Trulance vs placebo as early as week 1, which remained consistent through week 12 (Trulance n=430, placebo n=440).5
This analysis was not adjusted for multiplicity and should be interpreted with caution.
Trulance improved straining scores
Improved straining scores vs placebo throughout the treatment period2
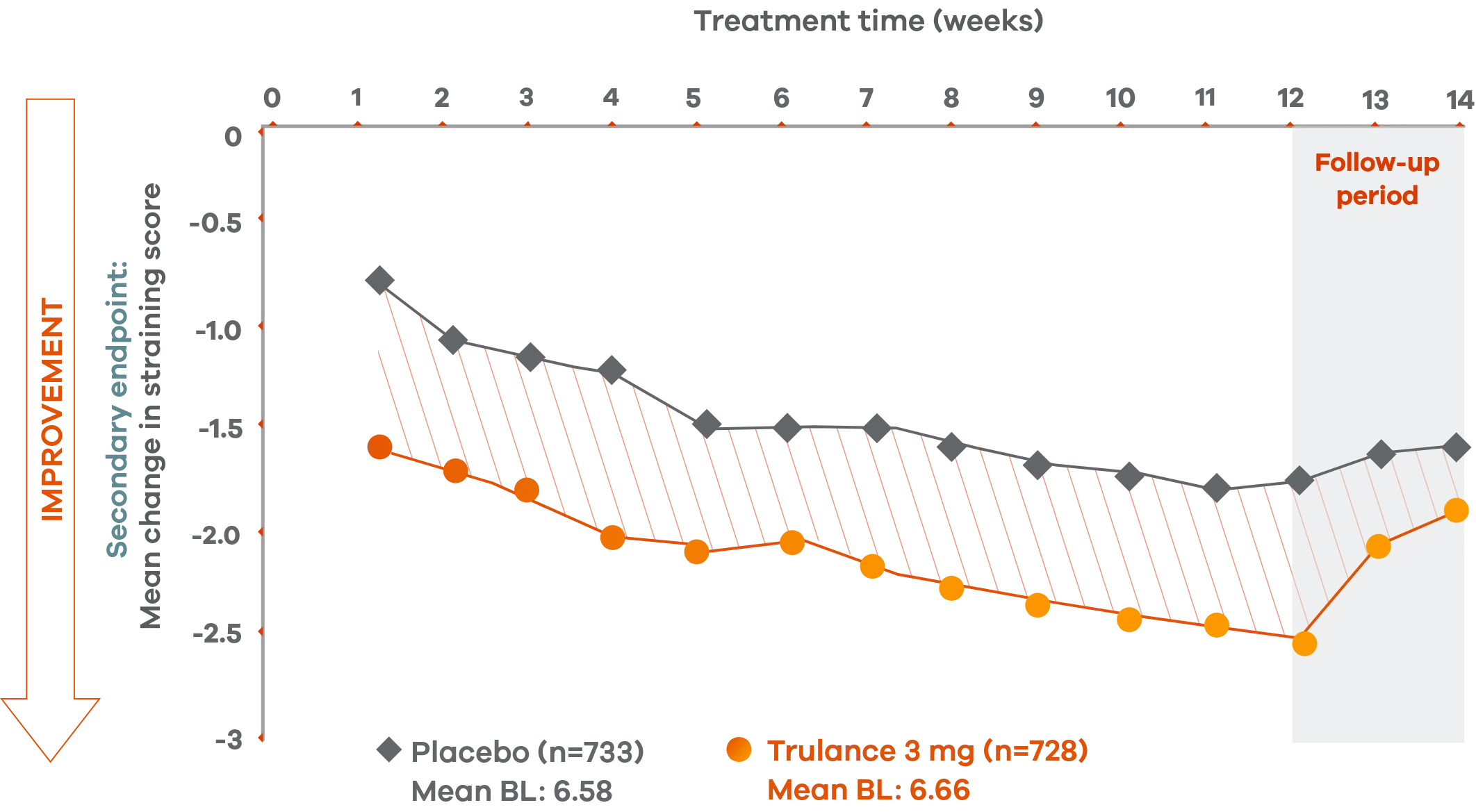

An integrated analysis of IBS-C Studies 3 and 4. The severity of straining during bowel movement was assessed on an 11-point numeric rating scale from 0 (none) to 10 (worst possible).2
This analysis was not adjusted for multiplicity and should be interpreted with caution.
Reduction in strainingp scores in patients with CIC4,7
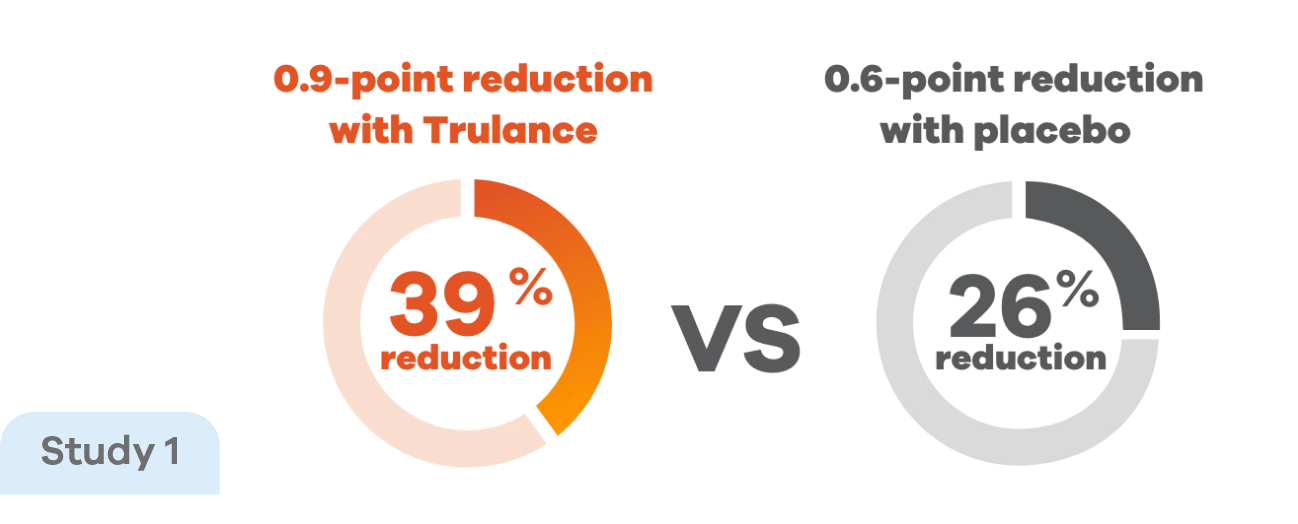

Improvements in straining score were observed early in treatment and were maintained throughout the study, with Trulance reducing straining scores by 39% vs 26% with placebo.4
Study 2 demonstrated less straining in Trulance-treated patients, beginning as early as Week 1 and remaining consistent through Week 12 vs placebo, –0.89 vs –0.61.7
pStraining was reported in Daily Symptom Diary using 5-point Likert Scale: 0=none; 4=very severe.4
This analysis was not adjusted for multiplicity and should be interpreted with caution.
Indication
Trulance (plecanatide) 3 mg tablets are indicated in adults for the treatment of Chronic Idiopathic Constipation (CIC) and Irritable Bowel Syndrome with Constipation (IBS-C).
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
Trulance® is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile mice, administration of a single oral dose of plecanatide caused deaths due to dehydration. Use of TRULANCE should be avoided in patients 6 years to less than 18 years of age. The safety and effectiveness of TRULANCE have not been established in patients less than 18 years of age.
TRU.0041.USA.24


 Bloating
Bloating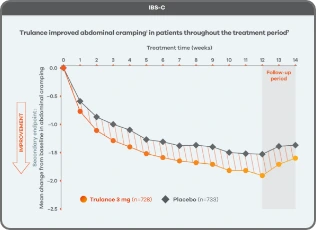 Cramping
Cramping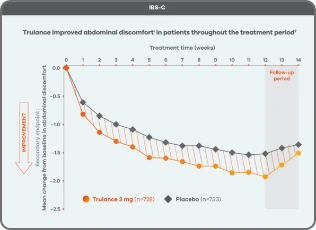 Discomfort
Discomfort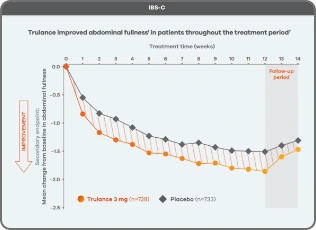 Fullness
Fullness